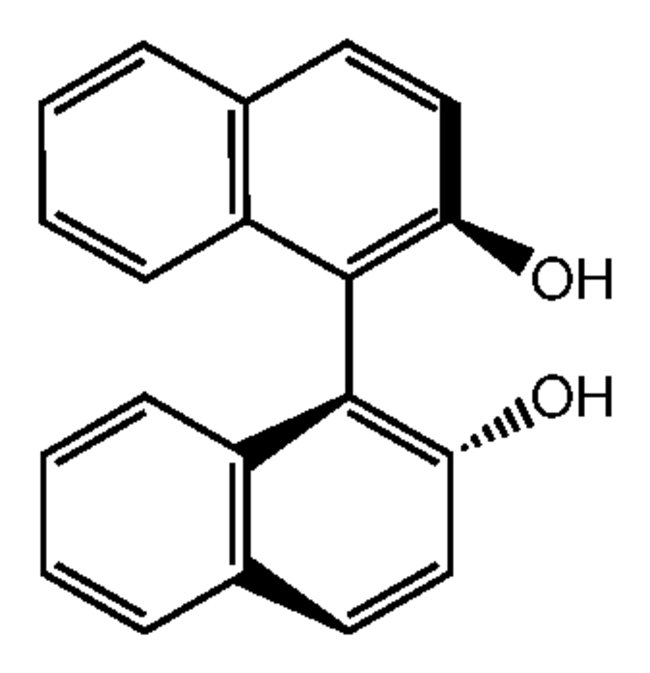
(R)-( )-1,1'-Bi(2-naphthol), 99%, Each
$ 51.98
|
|
Details:
It is used in biosynthetic preparation for enantioselective oxidation of naphthols to binaphthyldiols with horseradish peroxidase catalyst. A chiral auxiliary used in the catalytic asymmetric oxidation of sulfides to sulfoxides. Chiral lanthanide triflates formed from binaphthol serve as catalysts for asymmetric Diels-Alder reactions. Derivatives of binaphthol have recently found use in asymmetric Claisen rearrangements and asymmetric epoxidations.5 The lithium aluminum hydride derivative of these diols (BINAP-H) has been used extensively for the reduction of ketones. Chiral binapthol imminium salt precursor. Salts were used for an asymmetric epoxidation of olefins.
Additional Information
| SKU | 10098452 |
|---|---|
| UOM | 1g |
| UNSPSC | 12352100 |
| Manufacturer Part Number | L0830503 |
| CAS Number | 18531-94-7 |
| Is Hazardous | Yes |
| HS Code | 2907159000 |
|---|---|
| UN Number | UN 2811 |
| Proper Shipping Name | (R)-(+)-1,1'-Bi-2-naphthol |
| Packaging Group | PG III |
| Hazardous Class | 6.1 |
| Label |  |
| Molecular Formula | C20H14O2 |
| EC Number | 606-048-4 |
| HIN | 66 |
| Hazard Statement | H301-H315-H319 |
| Precautionary Statements | P201-P202-P264-P270-P280-P301+P310+P330-P302+P352+P332+P313+P362+P364-P305+P351+P338+P337+P313-P308+P313-P405-P501-P261-P280a-P304+P340-P501a-P280h-P309-P310-P301+P310-P305+P351+P338 |
| Risk Statements | 25-36-36/37/38 |
| GHS | GHS06 |
| GHS (Pictogram) |  |
| Safety Statements | 26-45-24/25-36 |
| Hazard Code | T,Xi |
| Signal Word | Danger |
