CD105 (Endoglin) Mouse anti-Human, Unlabeled, Clone: 35, BD, Mouse Monoclonal Antibody, Each
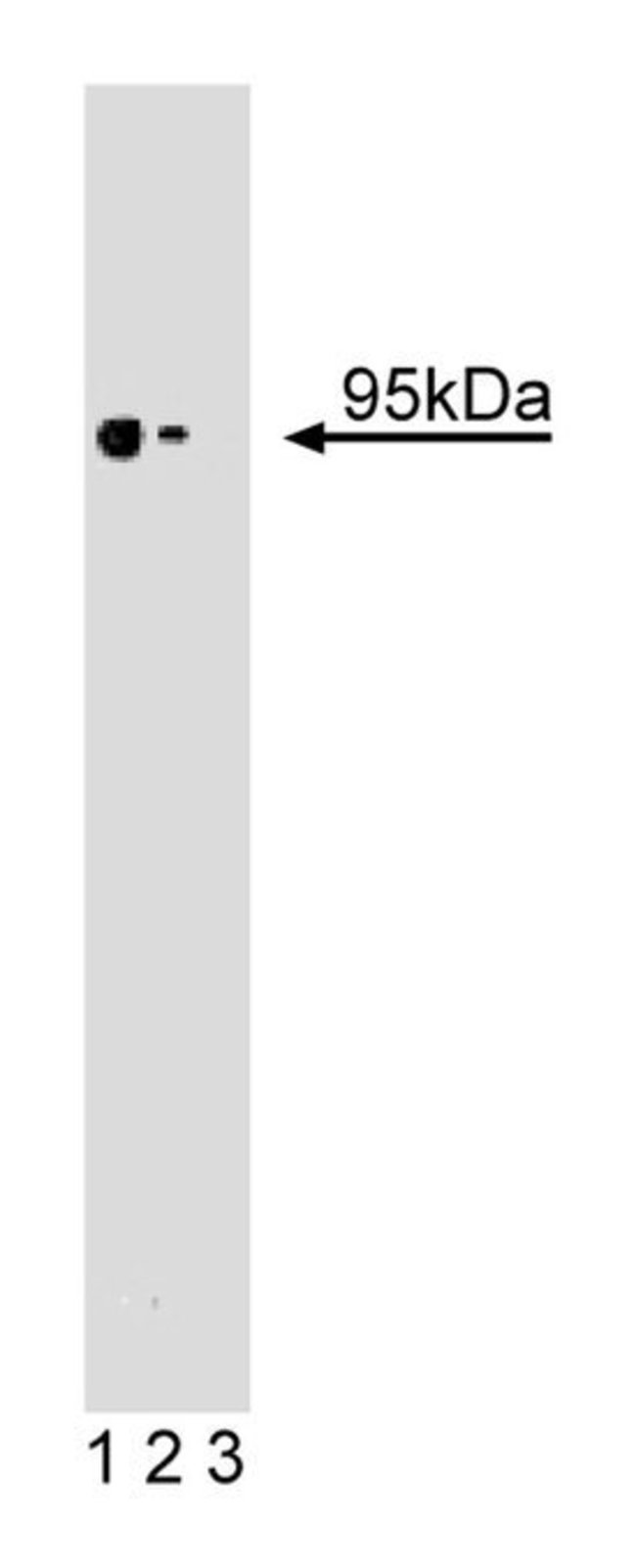
Details:
Endoglin (CD105), a major glycoprotein of human vascular endothelium, is a type I integral membrane protein with a large extracellular region, a hydrophobic transmembrane region, and a short cytoplasmic tail. It also contains an RGD tripeptide that may be important for cellular adhesion. There are two forms of endoglin (S-endoglin and L-endoglin) that differ in the length of their cytoplasmic tails. However, the isoforms may have similar functional activity. When overexpressed in fibroblasts, both form disulfide-linked homodimers via their extracellular domains. Endoglin binds TGF-β1 and TGF-β3 by associating with TGF-β type II receptor and binds BMP-7 by associating with activin type II receptor. Thus, endoglin is an accessory protein of multiple TGF-β superfamily kinase receptor complexes. Loss of function mutations in the human endoglin gene cause hereditary hemorrhagic telangiectasia, which is characterized by vascular malformations. Deletion of endoglin in mice leads to death due to defective vascular development. Thus, endoglin is an endothelial specific cell surface protein that may regulate angiogenesis through interactions with TGF-β superfamily kinase receptors.Host Species: MouseClone: 35Isotype: IgG1 κSpecies Reactivity: HumanImmunogen: Human S-Endoglin aa. 24-144Formula Weight [Chemical]: 95kDaImmunofluorescence, Western Blotting
Additional Information
| SKU | 10135341 |
|---|---|
| UOM | Each |
| UNSPSC | 12352203 |
| Manufacturer Part Number | 611315 |
