Dynactin p50 Mouse, Unlabeled, Clone: 25, BD, Mouse Monoclonal Antibody, Each
Details:
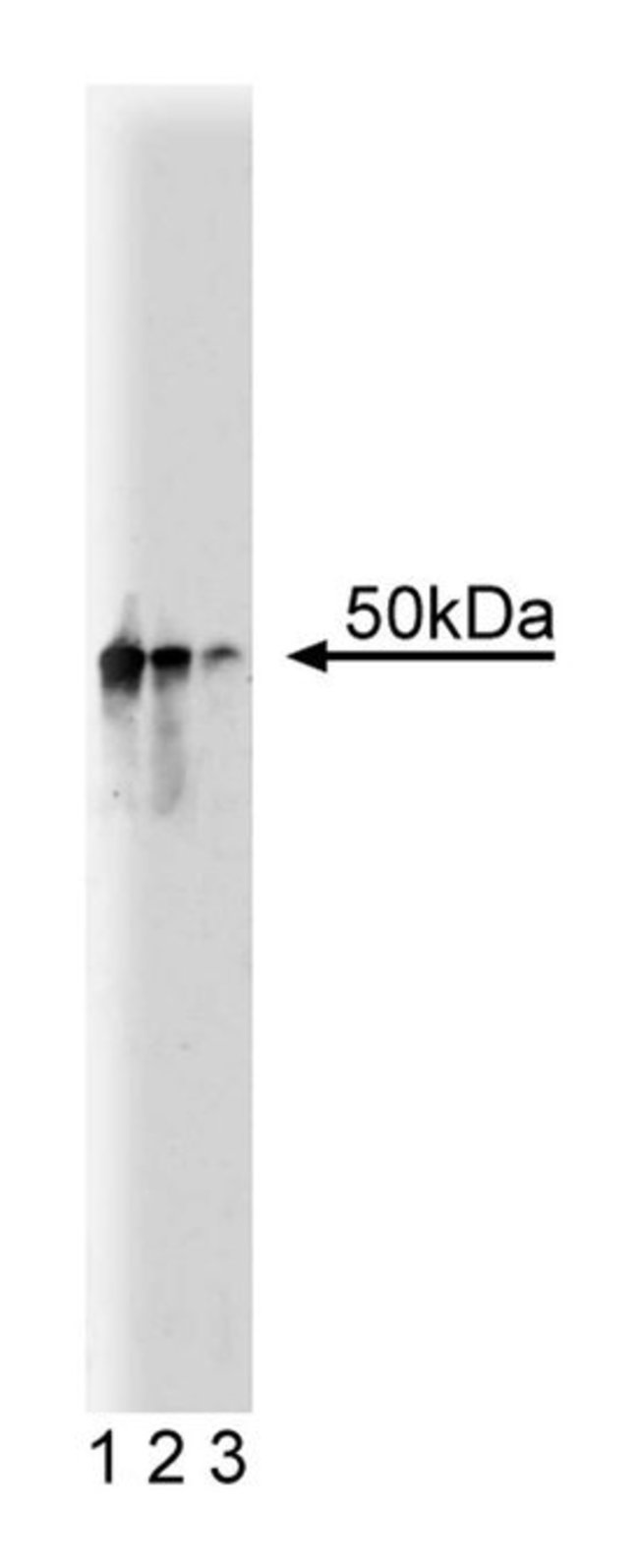
Dyneins are ubiquitous, multimeric proteins that are responsible for minus end-directed microtubule-based organelle transport. They are divided into two classes, cytosolic and axonemal. The activity of cytosolic dynein is regulated by the multiprotein dynactin complex with which it co-purifies. Dynactin is composed of at least nine polypeptides with p50 being the second most abundant subunit. The complex consists of an F-actin-like core filament of actin-related protein Arp1, a heterodimer that acts as a cap at one end, and a 62kDa subunit at the other end. p150[glued] is the largest subunit of the complex. The precise function of dynactin remains to be determined, but it may be recruited to prometaphase kinetochores to assist in alignment and spindle organization during mitosis. Additionally, dynactin localizes at the tail of cytosolic dynein and activates dynein-mediated vesicle movement. Therefore, dynactin may function to anchor or target dynein to the kinetochore during mitosis.
Additional Information
| SKU | 10135237 |
|---|---|
| UOM | Each |
| UNSPSC | 12352203 |
| Manufacturer Part Number | 611002 |
