gp91[phox] Mouse, Unlabeled, Clone: 53, BD, Mouse Monoclonal Antibody, Each
Details:
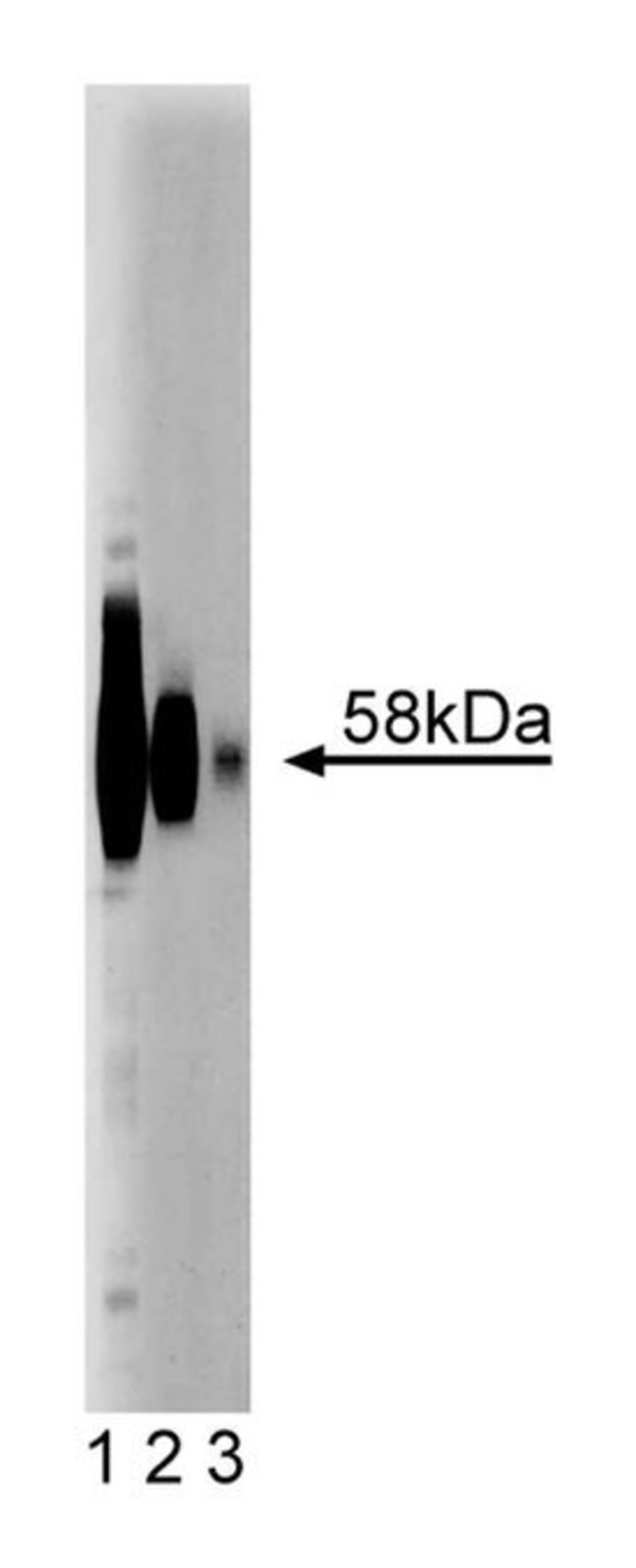
Although dormant in resting granulocytes, macrophages, and B lymphocytes, the neutrophil respiratory burst oxidase (NADPH-oxidase) generates superoxide and secondary oxygen-derived toxic products in response to bacteria or a variety of soluble stimuli. It is an integral membrane cytochrome, b558, which consists of two subunits, gp91[phox] and p21[phox]. Upon stimulation, cytochrome b558 forms a complex with cytosolic proteins, p67[phox], p47[phox], p40[phox], and rac2 and produces superoxide anions in a NADPH-dependent manner. In chronic granulomatous disease (CGD), severe recurrent bacterial and fungal infections result from defective NADPH-oxidase activity. The majority of CGD cases are caused by a defective gp91[phox] gene. gp91[phox], implicated as a docking site for p47[phox], is membrane glycoprotein with multiple N-terminal hydrophobic domains and a hydrophilic C-terminus. The expression of gp91[phox] is restricted to terminally differentiated phagocytes and B lymphocytes. This cell type- and developmental stage-specific expression may be controlled by transcriptional activators, such as PU.1 and YY1, and transcriptional repressors, such as CCAAT displacement protein (CDP). Endogenous mouse gp91phox shows 58kDa band, instead of 91kDa observed in human. Both mouse and human deglycosylated gp91 are 54kDa. This difference may be due to less glycosylation sites in the mouse sequence.Host Species: MouseClone: 53Isotype: IgG1Species Reactivity: MouseImmunogen: Mouse gp91[phox] aa. 450-556Formula Weight [Chemical]: 58kDaImmunofluorescence, Western Blotting
Additional Information
| SKU | 10135380 |
|---|---|
| UOM | Each |
| UNSPSC | 12352203 |
| Manufacturer Part Number | 611414 |
