GS28 Mouse, Unlabeled, Clone: 1, BD, Mouse Monoclonal Antibody, Each
Details:
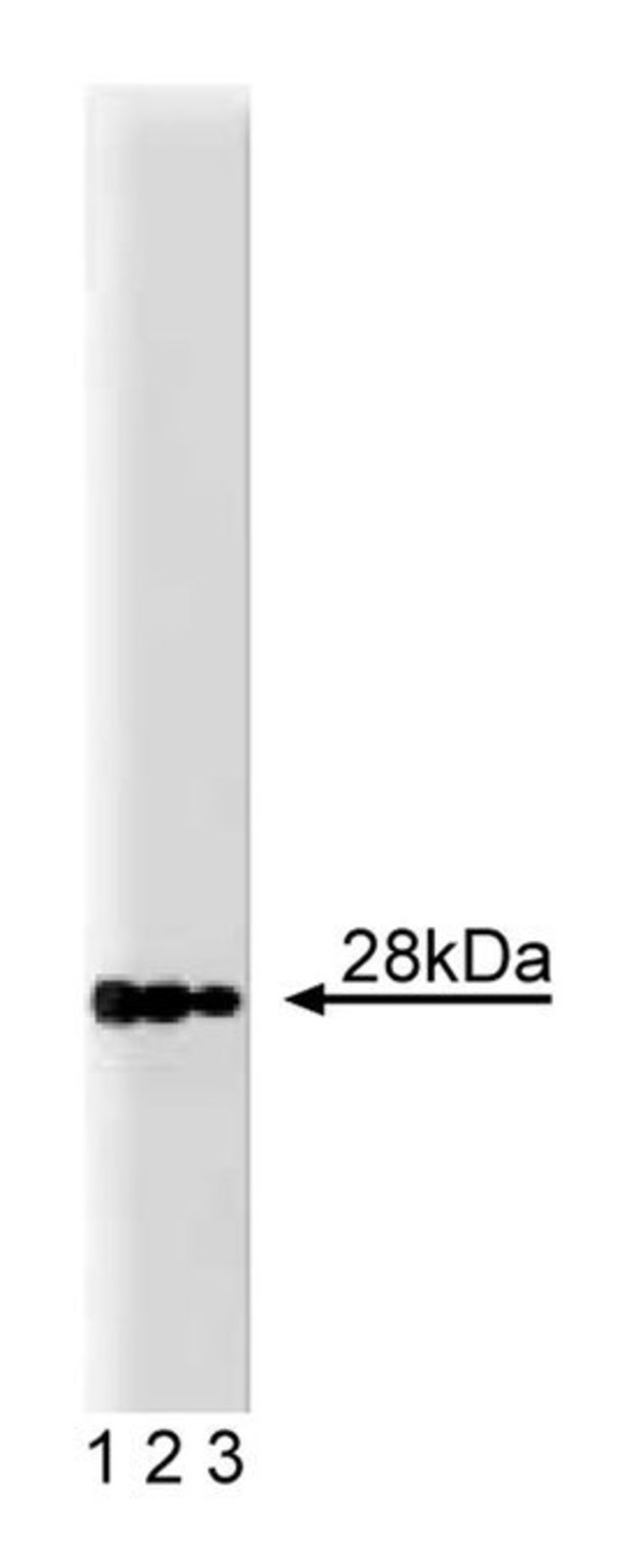
Most vesicular fusion events require the function of N-ethylmaleimide-sensitive factor (NSF) and the soluble NSF attachment proteins (SNAPs). Their function is dependent upon the SNAP receptor (SNARE) family of proteins. The specificity of vesicle docking and fusion is mediated by specific interactions between v-SNAREs on vesicles and t-SNAREs on target membranes. GS28 (Golgi SNARE with a size of 28kDa) is a SNARE that associates with the cis-Golgi and participates in trafficking between the ER and the Golgi and between Golgi compartments. The majority of GS28, the first 230 and 250 aa, is thought to be anchored to the membrane via the C-terminal hydrophobic tail, which is formed by the remaining 20 aa. GS28 and syntaxin 5, another SNARE, exist as a protein complex in the Golgi and this complex is important for the function of both proteins in ER-Golgi transport. The GS28/syntaxin 5 complex can be dissociated by α-SNAP and NSF. In addition, GS28 is thought to interact with α-SNAP when the GS28/syntaxin 5 complex is dissociated. Thus, GS28 is a SNARE protein that mediates, in complex with syntaxin 5, transport within the Golgi and between Golgi and ER.Host Species: MouseClone: 1Isotype: IgG2aSpecies Reactivity: MouseImmunogen: Rat GS28 aa. 3-108Formula Weight [Chemical]: 28kDaImmunofluorescence, Western Blotting
Additional Information
| SKU | 10135299 |
|---|---|
| UOM | Each |
| UNSPSC | 12352203 |
| Manufacturer Part Number | 611184 |
