Iron VACUettes Refill, Each
|
|
Details:
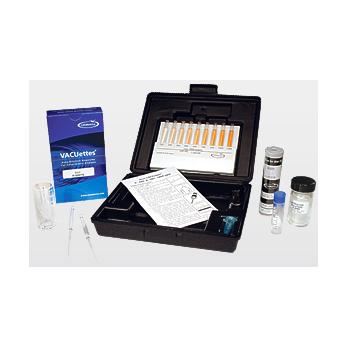
Iron is present in nature in the form of its oxides, or in combination with silicon or sulfur. The soluble iron content of surface waters rarely exceeds 1 mg/L, while ground waters often contain higher concentrations. The National Secondary Drinking Water Standard for iron is 0.3 mg/L, as iron concentrations in excess of 0.3 mg/L impart a foul taste and cause staining. High concentrations in surface waters can indicate the presence of industrial effluents or runoff.Iron contamination in oil field brines are typically a result of corrosion processes of iron-containing metallic components and equipment. Accumulation of insoluble iron salts in a brine completion fluid can result in substantial formation damage and can significantly affect the productivity of an oil well. Quantifying total iron in brine is critical.The Phenanthroline Method (total & soluble; total & ferrous)References: APHA Standard Methods, 23rd ed., Method 3500-Fe B - 1997. ASTM D 1068-77, Iron in Water, Test Method A. J.A. Tetlow and A.L. Wilson, “The Absorptiometric Determination of Iron in Boiler Feed-water”, Analyst. Vol. 89, p. 442 (1964).With the Phenanthroline Method, ferrous iron reacts with 1,10-phenanthroline to form an orange-colored chelate. To determine total iron, thioglycolic acid solution is added to reduce ferric iron to the ferrous state. The reagent formulation minimizes interferences from various metals. Results are expressed as ppm (mg/L) Fe.Kit comes in a plastic case and contains everything needed to perform 30 tests (except distilled water): Refill, Low and High Range Comparators, Activator Solution, dilutor snapper cup, small micro test tube, 5mL micro test tube, and instructions.Range: 0-120 & 120-1200 ppmMDL: 20 ppmMethod: Phenanthroline (total & soluble)Note: Kit contains only enough Activator Solution to perform approximately 7 total iron tests.
Additional Information
| SKU | 5336132 |
|---|---|
| UOM | Each |
| UNSPSC | 41116105 |
| Manufacturer Part Number | R-6001B |
