MLH1 with control Mouse anti-Human, Unlabeled, Clone: G168-15, BD, Mouse Monoclonal Antibody, Each
Details:
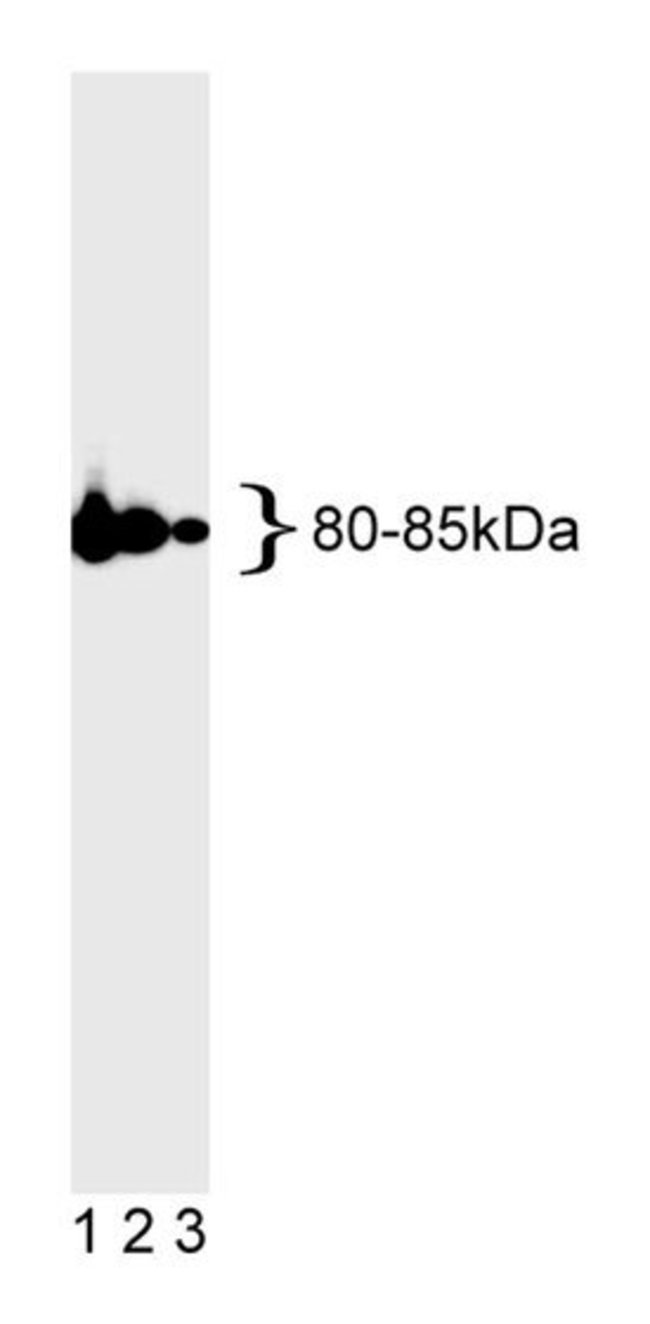
The repair of mismatched DNA is essential to maintaining the integrity of genetic information over time. In bacteria the DNA repair process is accomplished by the MutL, MutH, and MutS proteins. The MutS protein initially recognizes and binds to mismatched DNA. Following this, MutH, an endonuclease, and MutL form a complex with MutS and carry out an excision repair mechanism. When bacteria are deficient in one of these enzymes a mutator phenotype arises characterized by genetic instability. The important role played by DNA repair enzymes is emphasized by the fact that they are highly conserved from bacteria to yeast to mammals. In yeast the proteins are called MutS homolog 2 (MSH2), MutL homolog (MLH1), and PMS1 which is also a homolog of MutL. MSH2 is involved in the initial recognition of mismatched nucleotides during the replication mismatch repair process. It is thought that after MSH2 binds to a mismatched DNA duplex it is joined by a heterodimer of MLH1 and PMS1 which together help facilitate the later steps in mismatch repair. Biochemical studies of the human homologs of DNA mismatch repair enzymes MLH1, PMS2, and MSH2 indicate that human MSH2 protein can bind mispaired DNA, and that human MLH1 and PMS2 can exist as a heterodimer. These and other studies support the conservation of eukaryotic DNA mismatch repair mechanisms. The G168-15 antibody recognizes human MLH1 (80-85 kDa). Full-length human recombinant MLH was expressed as a fusion protein, affinity purified, and used as immunogen.
Additional Information
| SKU | 10133228 |
|---|---|
| UOM | Each |
| UNSPSC | 12352203 |
| Manufacturer Part Number | 551091 |
