53 Mouse, Unlabeled, Clone: PAB 240, BD, Mouse Monoclonal Antibody, Each
Details:
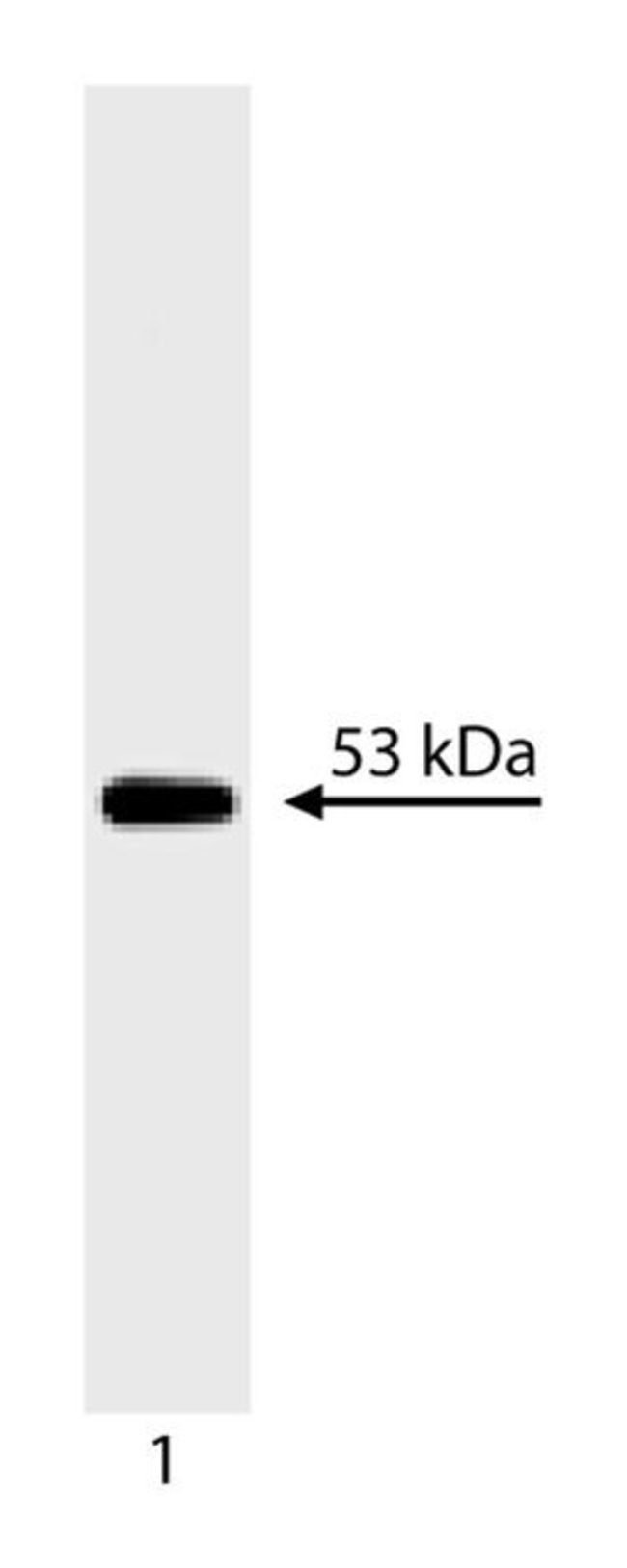
53 is a nuclear phosphoprotein which acts as a tumor suppressor by providing a cell cycle checkpoint for DNA damage during S-phase. Mutations in wildtype p53 can indirectly alter the DNA binding and transcription factor activity of p53. By altering expression of genes normally regulated by p53, these mutations can result in both a loss of tumor suppressor function and a gain of oncogenic function. The majority of mutations in the p53 gene are missense mutations which alter the identity of an amino acid. These mutations may alter the conformation and thus increase the stability of the mutant p53 protein. p53 is expressed in all vertebrate species examined. p53 may be overexpressed in transformed cell lines, where it forms complexes with viral oncogenes including SV40 large T antigen and the adenovirus protein, E1B. p53 migrates at ∽53kDa on SDS-PAGE. PAb 240 reacts with a conformational epitope between amino acids 156 and 214 of native p53. As such, Pab 240 recognizes only certain mutant forms of p53, as determined by immunoprecipitation. It detects both mutant and wildtype p53 in western blot analysis and immunohistochemistry of frozen tissue sections. It is thought that p53 mutations exert a common conformational change which results in expression of the Pab 240-specific epitope on mutant p53 molecules. A recombinant fusion protein of p53 sequence including amino acids 14-289 was used as immunogen.Host Species: MouseClone: PAb 240Isotype: IgG1Species Reactivity: Human, Mouse, Rat, Hamster, Monkey, Bovine, ChickenImmunogen: Human p53 aa. 14-289Formula Weight [Chemical]: 53kDaImmunohistochemistry (Frozen), Immunoprecipitation, Western Blotting
Additional Information
| SKU | 10133579 |
|---|---|
| UOM | Each |
| UNSPSC | 12352203 |
| Manufacturer Part Number | 554166 |
