67 (phox) Mouse, Unlabeled, Clone: 29, BD, Mouse Monoclonal Antibody, Each
Details:
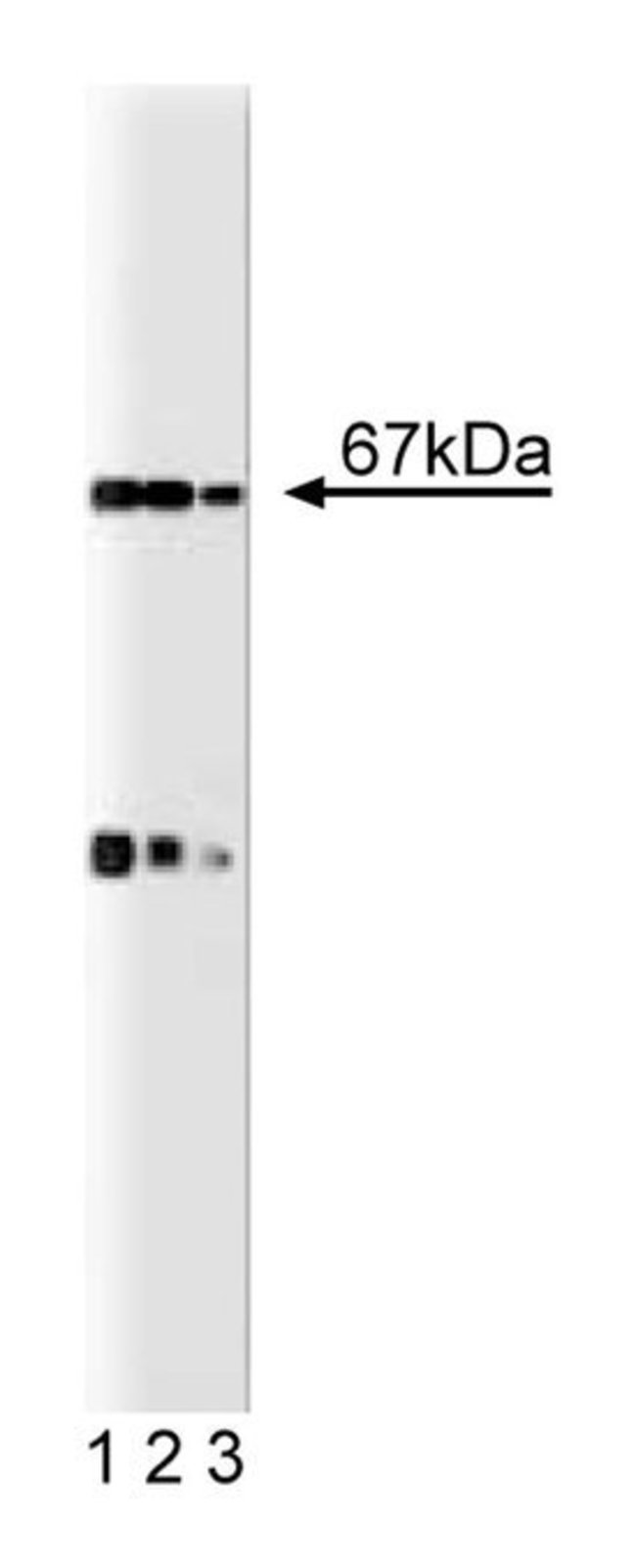
The neutrophil respiratory burst oxidase (NADPH-oxidase) generates superoxide and secondary oxygen-derived toxic products in response to bacteria or a variety of soluble stimuli. The active site of this enzyme is located in an integral membrane cytochrome, b558, that consists of the two subunits gp91 [phox] and p21 [phox]. Superoxide production depends on the formation of a complex that includes p67 [phox], p47 [phox], and the GTP-binding protein Rac. Upon activation, these proteins translocate from the cytosol to the membrane where they assemble with b558 and induce oxidase activity. p67 [phox] contains two SH3 domains and binds, via its C-terminal SH3 domain, to the proline rich region of p47 [phox]. This binding allows p67 [phox] to indirectly associate with the oxidase. It is thought that the phosphorylated forms of p67 [phox] and p47 [phox] interact and that the phosphorylation of p67 [phox] is regulated by both PKC-dependent and independent pathways. Although the role of p67 [phox] in electron flow control is poorly understood, it is thought that it regulates the transfer of electrons from NADPH to reduce flavin.Immunofluorescence, Western Blotting
Additional Information
| SKU | 10135185 |
|---|---|
| UOM | Each |
| UNSPSC | 12352203 |
| Manufacturer Part Number | 610913 |
