70 [S6K] Mouse, Unlabeled, Clone: 16, BD, Mouse Monoclonal Antibody, Each
Details:
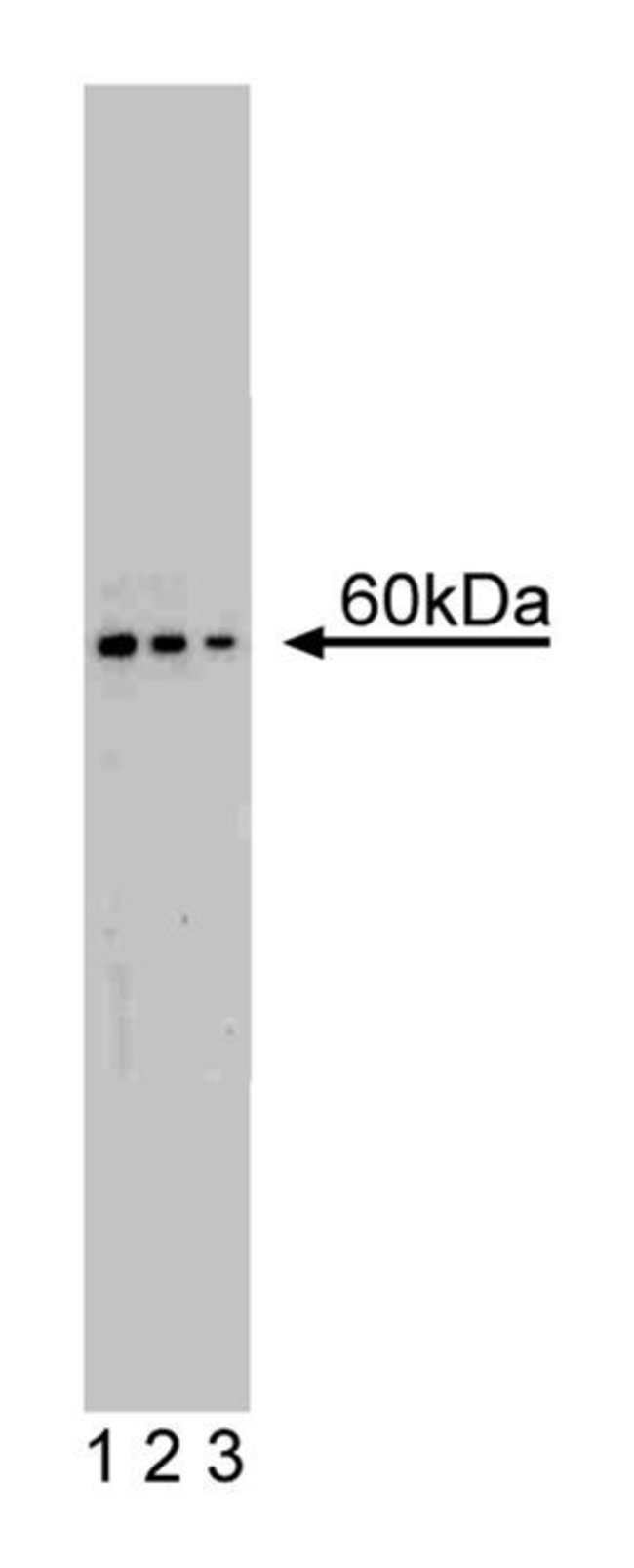
Increased protein synthesis, an obligatory step in cell proliferation, is regulated by the phosphorylation of a number of translational components, such as ribosomal protein S6. p70 S6 Kinase (p70 [S6K]), a cytosolic kinase that is primarily responsible for S6 phosphorylation, belongs to a family of at least six S6 kinases. p70 [S6K] and p85 [S6K] are two isoforms of the same kinase. Although they are encoded by the same gene, p80 [S6K] contains a 23 amino acid N-terminal extension that serves as a nuclear localization sequence. These kinases are located in a p21 ras-independent signaling pathway and are regulated by Ser/Thr phosphorylation in response to mitogenic stimulation. Activation of p70 [S6K] requires sequential phosphorylations within the autoinhibitory domain and at Thr 389. p70 [S6K] associates with PDK-1 and PKC? to form a possible PI3-K signaling complex. The immunosuppressive agent rapamycin forms an intracellular complex with FKBP12 and FRAP (RAFT1) that inhibits phosphorylation of p70 [S6K] at Thr 389 and, in turn, prevents enzyme activation. Thus, p70 [S6K] is an important regulator of cell proliferation and a potential target of agents that modify the immune and proliferative responses. p70 [S6K] has been reported to be observable migrating within a range of 60-70 kD by western blotting.Host Species: MouseClone: 16Isotype: IgG1Species Reactivity: RatImmunogen: Rat p70 [S6K] aa. 2-121Formula Weight [Chemical]: 60-70kDaImmunofluorescence, Western Blotting
Additional Information
| SKU | 10135325 |
|---|---|
| UOM | Each |
| UNSPSC | 12352203 |
| Manufacturer Part Number | 611260 |
