Syntaxin 4 Mouse anti-Human, Unlabeled, Clone: 49, BD, Mouse Monoclonal Antibody, Each
Details:
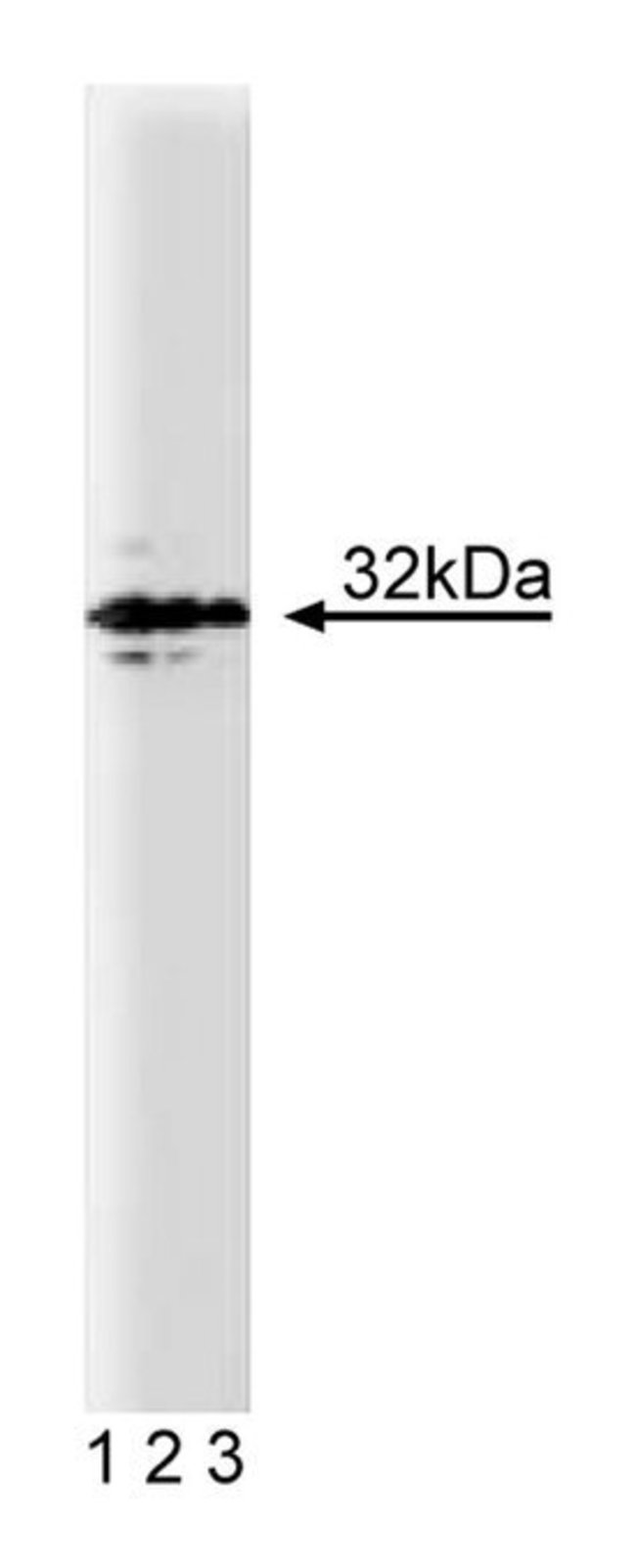
Signal transmission between neurons is regulated by the release of neurotransmitters at the synapse. This process is controlled by a complex pathway of membrane trafficking in the presynaptic nerve terminal, which leads to membrane fusion and subsequent neurotransmitter secretion. Syntaxin is involved in three important protein complexes that modulate this process: syntaxin and n-sec1, syntaxin, VAMP and SNAP-25, and syntaxin, VAMP, SNAP-25, αSNAP, and NSF (20S complex). A model has been proposed to explain docking, activation, and fusion of synaptic vesicles with donor membranes. This model suggests that VAMP/synaptobrevin and synaptotagmin (vSNARE) on the synaptic vesicle, and SNAP-25 and syntaxin (tSNAREs) on the plasma membrane, interact to form a 7S complex. It appears that syntaxin associates with -sec1 prior to and/or during the formation of the 7S complex. Two additional soluble proteins, αSNAP and NSF, associate with this complex as synaptotagmin releases from the complex. The resulting 20S complex contains syntaxin, SNAP-25, VAMP, αSNAP, and NSF. A large region including the N-terminus is involved in binding n-sec1 while both VAMP and SNAP-25 bind within residues 199-288 of syntaxin.Immunofluorescence, Immunoprecipitation, Western Blotting
Additional Information
| SKU | 10134965 |
|---|---|
| UOM | Each |
| UNSPSC | 12352203 |
| Manufacturer Part Number | 610439 |
